How AI Healthcare Startups tackle compliance challenges

AI healthcare startups face a paradox: move fast, but stay compliant. Here are 5 ways leading startups are turning compliance into a growth enabler.
Artificial intelligence (AI) is transforming healthcare — from predictive analytics in patient care to advanced diagnostic imaging and virtual health assistants. Startups are at the forefront of this innovation, rapidly developing solutions that promise to improve accuracy, reduce costs, and personalize treatment.
Yet, one of the biggest hurdles AI healthcare startups face is compliance. Unlike consumer apps or retail software, healthcare solutions operate in one of the most heavily regulated industries worldwide. Every line of code that touches patient data is subject to scrutiny, and failure to comply with regulations doesn’t just lead to fines — it can destroy trust and stall funding rounds.
For example:
- In the U.S., startups must comply with HIPAA (Health Insurance Portability and Accountability Act), which enforces strict controls on how patient health information (PHI) is stored, shared, and protected.
- In Europe, the GDPR (General Data Protection Regulation) governs how sensitive patient data is processed, with penalties reaching up to €20 million or 4% of annual revenue.
- In Asia-Pacific markets like Singapore and Vietnam, laws such as PDPA (Personal Data Protection Act) are increasingly aligning with global standards.
This creates a paradox for startups: they must move fast to innovate but carefully navigate regulatory landscapes that even large enterprises struggle with. Investors, healthcare providers, and patients are increasingly asking the same question: “How can startups ensure compliance without slowing down innovation?”
That’s the core of this discussion. In this blog, we’ll explore the key compliance challenges AI healthcare startups face, strategies they use to overcome them, and why outsourcing can be a smart path to staying compliant while scaling.
5 Compliance Strategies Every AI Healthcare Startup Must Know
I. What are key compliance challenges for AI Healthcare Startups?
Building an AI product for healthcare isn’t the same as creating a generic app — the stakes are higher, the risks are greater, and the regulatory landscape is much stricter. Below are the four biggest compliance challenges that AI healthcare startups must overcome:

1. Data Privacy & Security
Why it matters: Patient data is among the most sensitive forms of personal information. Mishandling it can lead to devastating consequences, from identity theft to medical fraud.
- HIPAA (U.S.): Requires startups to safeguard Protected Health Information (PHI) through encryption, access control, and audit trails.
- GDPR (EU): Goes further by mandating “data minimization” and explicit patient consent for any AI-based data processing.
- Emerging standards: In 2025, regulators in countries like Australia, Singapore, and Vietnam have aligned their privacy laws with global frameworks, making compliance a multi-jurisdictional challenge.
Real-world risk: In 2023, a telehealth startup in the U.S. faced a $7 million penalty for failing to encrypt patient medical histories — a costly reminder that compliance is not optional.
2. Regulatory Approval & Certification
AI healthcare startups that build diagnostic or clinical tools face the same regulatory hurdles as medical device companies.
- FDA Approval (U.S.): AI-based diagnostic apps must undergo FDA’s Software as a Medical Device (SaMD) framework. This can delay product launches by 12–24 months.
- CE Marking (Europe): Under the EU Medical Device Regulation (MDR), startups must demonstrate safety, clinical validation, and ongoing monitoring.
- TGA (Australia) & MHRA (UK): National health authorities now require AI startups to show evidence of fairness, explainability, and accuracy before entering the market.
Challenge: Unlike hardware devices, AI models are continuously learning. Regulators are still evolving guidelines on how to approve software that “changes” with new data.
3. Bias, Fairness & Ethical AI
AI is only as good as the data it learns from. Startups often face compliance risks if their models introduce algorithmic bias.
- Bias Example: If an AI diagnostic tool is trained mostly on Western patient datasets, it may underperform for Asian or African populations — leading to unequal healthcare outcomes.
- Ethical Standards: Organizations like the World Health Organization (WHO) and OECD have published frameworks emphasizing fairness, transparency, and human oversight.
- Compliance Burden: Startups must demonstrate not only technical accuracy but also ethical accountability.
Investor Insight: According to McKinsey (2024), 74% of healthcare executives said “ethical AI” will be a deciding factor in vendor selection by 2026 — meaning compliance in fairness is now a business driver.
4. Interoperability & Standards
Even if an AI solution is technically advanced, it won’t succeed unless it can integrate with existing healthcare systems.
- Standards like HL7 and FHIR: Startups must ensure their apps communicate seamlessly with Electronic Health Records (EHRs).
- Vendor lock-in: Hospitals are wary of startups that don’t comply with interoperability rules, since non-compliance can block information exchange.
- Cross-border issues: In Asia-Pacific, data exchange between public and private hospitals is often fragmented, making compliance with standards even harder.
Example: In 2025, the U.S. Office of the National Coordinator for Health IT (ONC) enforced stricter FHIR-based interoperability rules, with penalties for vendors blocking data exchange. This puts pressure on startups to build compliance-first from day one.
By blending proactive design, certifications, legal guidance, regulatory pilots, and outsourcing, AI healthcare startups are turning compliance from a liability into a market differentiator. Those who can show compliance maturity early often attract more funding and faster hospital partnerships.
III. Strategies AI Startups Use to Tackle Compliance
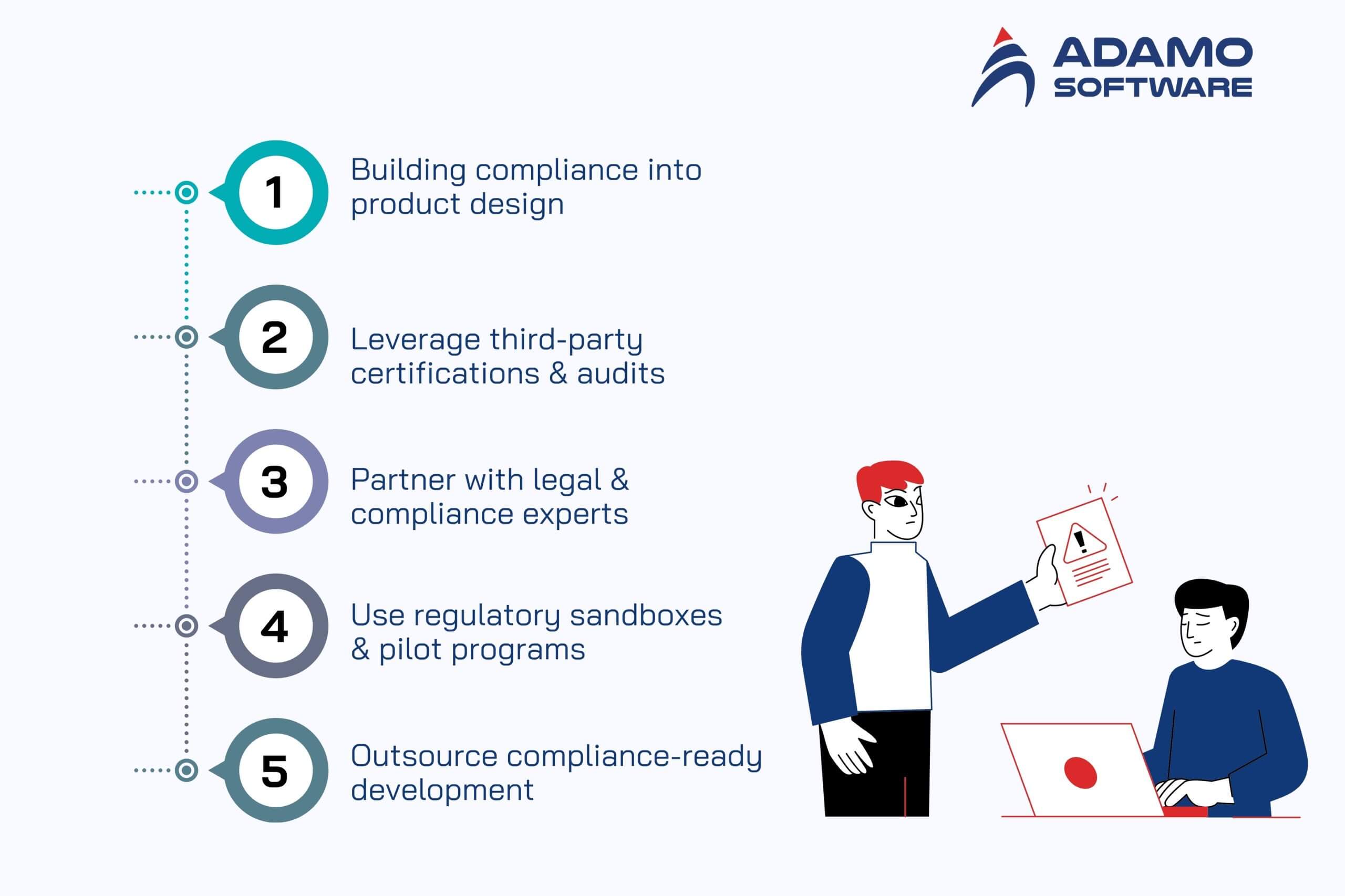
1. Building Compliance Into Product Design
For many successful AI healthcare startups, compliance isn’t treated as a final checklist item but as a foundation of product design. This approach, often called “privacy by design” and “security-first,” ensures that every feature is developed with regulations in mind. Encryption, anonymization, and access controls are implemented from the earliest development phases rather than bolted on later.
By prioritizing security at the architecture level, startups reduce risks and build trust with hospitals, insurers, and patients. A good example is Abridge, a U.S.-based medical transcription company, which embedded HIPAA compliance into its system from day one, enabling it to scale quickly within healthcare networks.
2. Leveraging Third-Party Certifications & Audits
Another strategy involves pursuing recognized certifications that signal credibility and maturity. Standards such as ISO 27001 and SOC 2 are widely respected and demonstrate that a startup has strong governance in place for information security and risk management.
In many cases, startups also take advantage of certified infrastructure providers like AWS, Azure, or Google Cloud, which already meet industry compliance benchmarks. These certifications are more than just regulatory shields — they act as sales enablers, making it easier for startups to secure contracts with health systems and attract investor confidence.
3. Partnering with Legal & Compliance Experts
Because most AI founders come from technical backgrounds, interpreting complex healthcare regulations can be daunting. To address this gap, startups increasingly partner with compliance experts, whether by hiring a Chief Compliance Officer, bringing consultants on board, or forming advisory panels with industry veterans.
These experts provide clarity on requirements like HIPAA, GDPR, or the FDA’s SaMD framework, ensuring that compliance obligations are correctly understood and integrated into product roadmaps. This proactive investment helps avoid costly missteps, such as releasing an AI tool that regulators later classify as a medical device requiring certification.
4. Using Regulatory Sandboxes & Pilot Programs
Regulatory sandboxes have emerged as a valuable tool for startups navigating compliance. These controlled environments, set up by regulators or public health systems, allow companies to test their AI solutions under supervision without the full burden of approval processes.
For example, the FDA’s Digital Health Software Precertification Program in the U.S. and the NHS AI Lab in the UK give startups opportunities to validate solutions in real-world scenarios while receiving regulatory guidance. Participating in such programs not only accelerates time-to-market but also builds credibility by showing that a startup is willing to work hand in hand with regulators.
5. Outsourcing Compliance-Ready Development
Finally, many AI healthcare startups are turning to outsourcing as a way to accelerate compliance while reducing costs. Partnering with software development firms that already have experience in HIPAA, GDPR, HL7, and FHIR ensures that compliance frameworks are built in from the start. This approach allows startups to focus on innovation while leveraging external expertise in regulated healthcare IT development.
Countries like Vietnam, India, and Eastern Europe are emerging as hubs for compliance-aware outsourcing, offering both cost advantages and specialized knowledge. For instance, firms like Adamo Software in Vietnam support startups with compliance-ready healthcare solutions, helping them bring products to market faster without overlooking critical legal and regulatory requirements.
Explore Our Tailor-made Software Development Solutions
We are confident in providing end-to-end software development services from fully-functioned prototype to design, MVP development and deployment.
IV. The role of Outsourcing in meeting Compliance Requirements
1. Overcoming resource constraints
AI healthcare startups often operate with small teams and limited budgets. Compliance can feel overwhelming when there’s constant pressure to innovate and scale. Outsourcing helps bridge this gap by providing access to specialists who understand both software development and healthcare regulations, allowing startups to focus on product innovation while external teams handle compliance-heavy tasks.
2. Access to compliance expertise
Outsourcing partners bring deep knowledge of global regulatory frameworks, including HIPAA, GDPR, HL7, FHIR, and ISO 27001. This ensures compliance isn’t an afterthought but is integrated into every step of the development process — from system design to testing and deployment. Startups that leverage this expertise reduce costly compliance risks and avoid delays in bringing products to market.
3. Faster audit and certification readiness
Preparing for audits, certifications, or regulatory submissions requires significant documentation and validation. Outsourcing firms can accelerate this process by supporting security audits, creating compliance reports, and aligning products with international benchmarks. This readiness not only satisfies regulators but also reassures investors and healthcare providers evaluating new AI solutions.
4. Global outsourcing hubs with healthcare IT strength
Countries such as Vietnam, Eastern Europe, and India have emerged as strong hubs for healthcare IT outsourcing. Vietnam, in particular, is known for its skilled developers, cost-effectiveness, and growing expertise in compliance-driven healthcare software. This makes it an attractive option for startups looking to scale quickly without sacrificing regulatory standards.
5. Adamo Software: A compliance-ready development partner
As a Vietnam-based software development company, Adamo Software has helped global healthcare startups navigate compliance challenges by delivering solutions aligned with HIPAA, GDPR, and international interoperability standards. With a track record in healthcare IT outsourcing, Adamo ensures that compliance is not a bottleneck but a built-in strength – enabling startups to scale confidently in regulated markets.
Ready to Outsource?
Get top-tier IT talent without the hassle. Contact us now!
V. Future outlook: Compliance as a competitive advantage
1. Compliance Will Become a Trust Signal
In the next decade, compliance will evolve from being just a regulatory requirement to a market differentiator. Healthcare providers and patients will choose AI solutions not only for their technical capabilities but also for their proven commitment to security, privacy, and fairness. Startups that showcase compliance maturity will earn stronger trust and loyalty.
2. Funding will favor compliance-ready startups
Investors are increasingly scrutinizing compliance as part of due diligence. By 2030, startups that embed HIPAA, GDPR, or EU AI Act frameworks into their operations early will be more attractive to venture capital firms, insurance companies, and healthcare providers. According to Deloitte’s 2025 outlook, compliance-readiness is projected to be one of the top three investment criteria for digital health startups.
3. AI governance frameworks will set global standards
Regulations like the EU AI Act, U.S. Department of Health and Human Services (HHS) AI guidelines, and global principles from organizations such as the OECD and WHO will create clearer rules for AI in healthcare. Startups that align with these governance models early will find it easier to expand internationally, while those that lag behind risk being locked out of critical markets.
4. Ethical AI will shape adoption
Regulations like the EU AI Act, U.S. Department of Health and Human Services (HHS) AI guidelines, and global principles from organizations such as the OECD and WHO will create clearer rules for AI in healthcare. Startups that align with these governance models early will find it easier to expand internationally, while those that lag behind risk being locked out of critical markets.
5. Outsourcing will evolve into compliance partnerships
The outsourcing model will also shift. Instead of being viewed as simple development partners, outsourcing vendors will become compliance co-pilots, helping startups adapt to evolving regulations across markets. This evolution positions countries like Vietnam — with its growing pool of healthcare IT talent — as critical partners in helping startups navigate the complex global regulatory landscape.
VI. Conclusion

Compliance is often seen as a roadblock for AI healthcare startups, but in reality, it is the foundation for long-term success. From protecting sensitive patient data and securing regulatory approval to eliminating bias and ensuring interoperability, compliance shapes whether an innovation is trusted, adopted, and scaled in real healthcare environments.
Startups that embed compliance into their DNA — through privacy-first design, certifications, expert guidance, regulatory sandboxes, and outsourcing partnerships — position themselves not only to meet legal obligations but also to gain a competitive advantage. As global regulations like the EU AI Act and HHS AI guidance reshape the healthcare AI landscape, compliance will increasingly act as a trust signal for investors, providers, and patients alike.
This is where outsourcing makes a real difference. By collaborating with compliance-ready development partners, startups can accelerate their go-to-market strategy without sacrificing quality or security. Adamo Software, as a Vietnam-based outsourcing company with proven expertise in healthcare IT, helps startups design, build, and scale AI solutions that meet international compliance standards such as HIPAA, GDPR, HL7, and FHIR. With the right partner, compliance becomes not a burden but a growth enabler.





