Medical device software development: What is it, types & benefits
Unlock the potential of medical device software development! Discover its types, key benefits, compliance factors, and a step-by-step guide to building successful solutions.
In the past decade, healthcare technology has advanced significantly. It ushered in a new era where software is an integral part of the development and functionality of medical devices. This includes diagnostic tools, treatment monitoring, and more. It can be said that medical device software is a fundamental component that is revolutionizing the healthcare industry.
It is undeniable that technology and medical devices have combined to revolutionize the design and implementation of healthcare solutions. This software integrates seamlessly into medical devices, surpassing traditional components. This integration has also led to creating more dynamic and adaptive solutions, promoting innovative and patient-centric approaches to healthcare.
Modern healthcare devices today are designed to be more compact and portable to accommodate technological advancements. It is more accessible outside clinical settings, reaching the patient’s home. Notably, these small medical devices have also proven highly effective and reliable, significantly impacting basic healthcare operations.
This Blog will provide a comprehensive overview of medical device software development. It will help you discover its key benefits, critical considerations during development, and the overall process of developing robust software. So, without further ado, let’s dive in!
I. What is Medical Device Software Development?
Simply put, medical device software is designed to perform essential tasks in medical devices and healthcare systems. This software plays a crucial role in various medical such as diagnosis, treatment, monitoring, and providing recommendations for medical use.
A typical example of medical device software you often encounter is a heart rate monitoring application that runs on a wearable health gadget to collect and check the patient’s heart rate data. The software processes the data and presents it in an intuitive, easy-to-read format.
With the increasing number of people using wearable devices, smart gadgets, and IoT-based systems, the demand for medical device software development continues to grow. The working mechanism of these software solutions may vary depending on the type of device and its specific functionality.
You can explore more about What is remote patient monitoring? Tech stacks and real-life examples here.
II. Types of medical device software
Today, many medical device manufacturers leverage advanced technology to design and develop their products. As a result, new types of medical device software continue to emerge. There are two basic types of medical device software on the market: embedded medical systems and Software as a Medical Device (SaMD).
1. Embedded Medical Systems
The first type is the embedded medical system. This system is designed to operate inside medical devices and is integrated directly into their hardware. Therefore, it allows the medical device to perform its functions independently without integrating with an external computer or server.
Embedded medical systems are often highly reliable and comply with strict safety regulations. These software components are integrated into the hardware and are often not independent. In addition, they are also linked to an important function in the core operations of the device.
Common embedded system medical device software configured with embedded code are:
- Pulse oximeter
- Smart biosensor
- Blood glucose meter
- Electronic blood pressure sensor
- Medical imaging equipment such as X-ray, MRI, CT
2. Software as a medical device (SaMD)
The second type is Software as a medical device (SaMD). Unlike embedded medical systems, this Software operates independently on various medical device hardware. It can include all programs that do not require dedicated medical devices.
SaMD can be used on mobile applications, cloud-based tools, or desktop applications specifically designed for medical systems. Therefore, these programs can be integrated with dedicated medical devices and embedded medical systems.
- Some examples of Software as a Medical Device (SaMD) include:
- Image analysis or patient scanning
- Remote ECG monitoring
- Medical data viewing applications
Ready to Outsource?
Discover how we can transform your business with expert IT solutions.
III. Benefits of Medical Device Software Development for Healthtech Startups
In the healthcare sector, medical device software is an important solution. It ensures higher operational efficiency and provides accuracy and safety in delivering healthcare services. In this section, let us take a detailed look at the benefits of medical device software development:
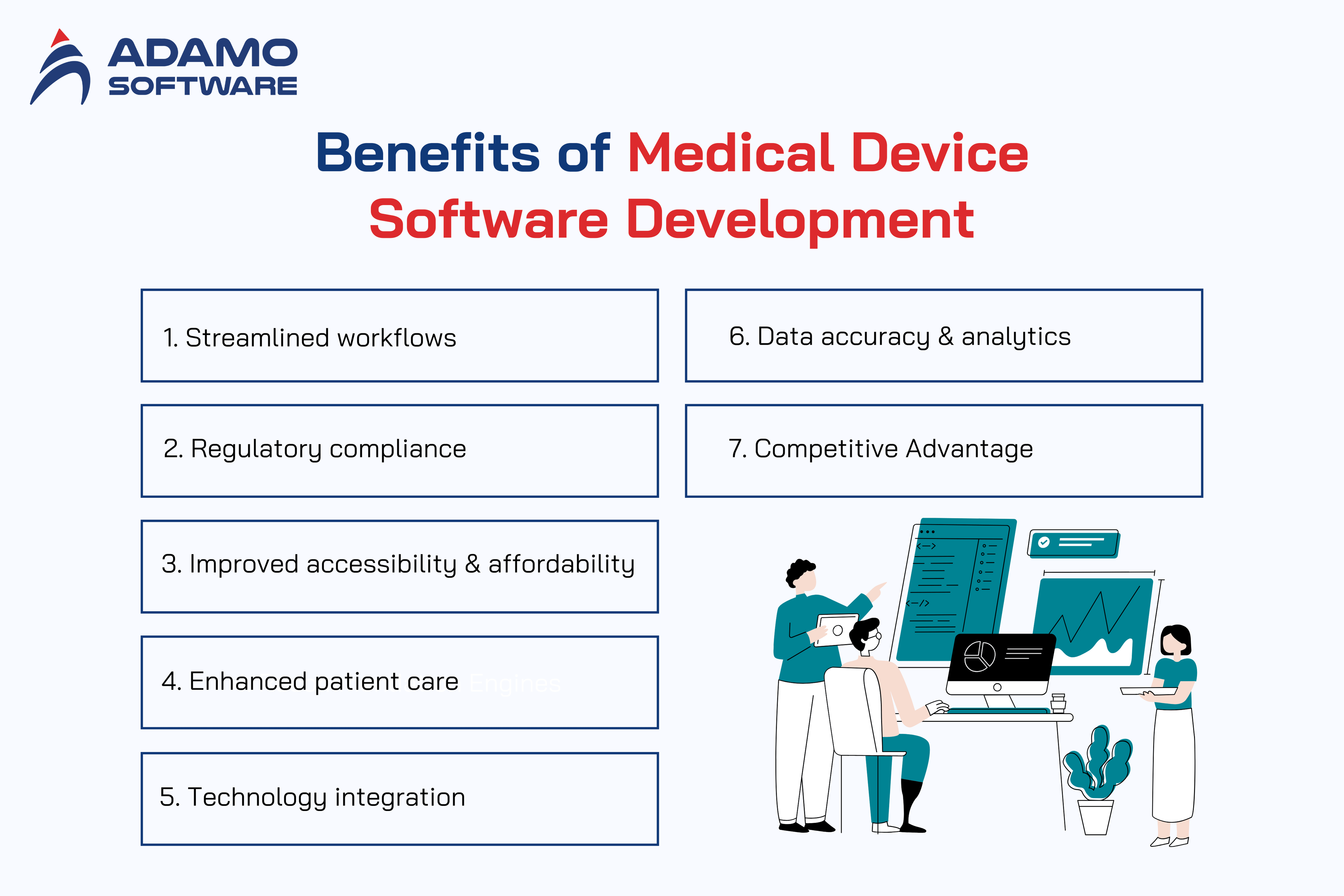
1. Streamlined Workflows
The first benefit of medical device software development is the creation of streamlined workflows. Integrating medical device software solutions helps to optimize employees’ workflow. At the same time, it improves work efficiency, reduces paperwork, and automates routine tasks. In addition, this shift allows healthcare professionals to shift their focus from administrative tasks to a more patient-centric approach.
2. Regulatory Compliance
By strictly adhering to healthcare industry regulations, medical device software development helps ensure compliance, minimize the risk of fines, and prioritize patient safety in the healthcare ecosystem.
Here are some important regional healthcare regulations that you should consider during your medical software development:
- United States (US): HIPAA, FDA, HITECH
- Japan: PMDA
- China: NMPA
- Australia: Data Protection Act, MHRA
- European Union (EU): MDR, GDPR, EMA
- India: CDSCO
- Canada: CMDR, PIPEDA
- Brazil: ANVISA
- International Compliance: ISO and IMDRF
3. Improved Accessibility and Affordability
Improved accessibility and affordability are a great benefit of medical device software. Medical device software development allows for expanding healthcare services beyond the conventional clinical setting. Furthermore, it improves accessibility and affordability for patients seeking medical assistance.
4. Enhanced Patient Care
Not to be left out of the list, the development of medical device software (MDS) helps improve patient care efficiency by enabling accurate diagnosis, advanced monitoring, and appropriate treatment planning. In addition, implementing this software contributes significantly to providing more efficient and effective care.
5. Technology Integration
Another notable benefit is technology integration. The implementation of advanced technology such as AI, IoT, and cloud computing helps service providers enhance the performance and functionality of medical devices. This creates advanced healthcare solutions that deliver superior patient care. In addition, this integration optimizes diagnostic accuracy and treatment precision. This revolutionizes healthcare services.
6. Data Accuracy and Analytics
Medical device software development ensures accurate data collection and complex analytics. It empowers healthcare practitioners to draw informed conclusions and helps them make informed decisions.
7. Competitive Advantage
Finally, a great benefit of medical device software development that you can leverage is the increased competitive advantage in the market. Therefore, businesses that invest in medical device development ensure a competitive advantage in the dynamic healthcare industry. By implementing innovative medical software, you can offer cutting-edge services and solutions to differentiate yourself from your competitors. This helps you strengthen your brand presence in the market.
IV. Factors to consider for medical device software development
Important factors must be considered before developing medical device software, and a strategic plan must be designed carefully. By carefully considering all of these factors, your business can create software that is safe, effective, and compliant with regulatory requirements.
1. Regulatory Compliance
The first and most important element in developing medical device software is to comply with strict regulatory standards, such as those established by the FDA. Compliance with these regulations ensures your medical device software’s safety, effectiveness, and quality. Additionally, regulatory compliance is essential to meeting market approval standards. It also provides patient safety by navigating complex legal and quality requirements.
2. Data Security and Privacy
The next important factor is implementing robust data security measures, including encryption and access controls. This helps protect sensitive patient information from being breached or accessed without authorization. Maintaining patient trust and complying with data protection laws such as HIPAA is also essential.
3. Usability and User Experience
Medical device software development should be user experience centric. It is important to ensure that the software is intuitive and easy to navigate for healthcare professionals and patients. Additionally, a well-designed and intuitive interface increases efficiency, reduces errors, and promotes seamless interaction between the user and the software. As a result, patient care becomes more efficient, and overall satisfaction improves.
4. Interoperability with Healthcare Systems
The next essential element is user interoperability. Developing medical device software that can communicate and integrate seamlessly with other devices in the healthcare ecosystem is fundamental. Not only does it facilitate seamless data exchange across multiple platforms, it also contributes to improved patient outcomes and more efficient healthcare delivery.
5. Risk Management
Any software development process has inherent risks. Therefore, thorough risk analysis processes are essential to identify potential hazards and mitigate the risks associated with the software. This may include comprehensive risk assessment, management, and mitigation strategies. Ensure that you always ensure patient safety and minimize potential harm.
6. Collaboration with Stakeholders
Engaging and partnering with stakeholders such as healthcare professionals, patients, and regulatory agencies is critical. It ensures that your medical device software consistently meets the exact requirements. It also helps to enhance the functionality and suitability of the software in real-world healthcare settings. This collaboration ensures that your software aligns with user needs and regulatory standards.
7. Testing and Validation
Another factor that you must consider during the medical device software development process is testing and validation. This is important to ensure that your software is bug-free, operates reliably, and meets established quality standards. Furthermore, these processes allow you to identify and resolve potential issues or errors before the software is deployed for actual use. Ensuring the efficiency and performance of the software.
8. Continuous Improvement and Maintenance
A final important factor is continuous maintenance and updates. In addition to developing medical device software, implementing continuous improvement strategies is essential to keep the software up to date and relevant to changing healthcare needs.
At the same time, it helps to keep your software free of problems and errors during use. Therefore, improvement is essential to address emerging challenges and maintain high performance and safety standards.
V. Step-by-step guide for successful Medical Device Software Development
To ensure your medical device software is compliant, high-quality, and efficient, you can follow these steps:
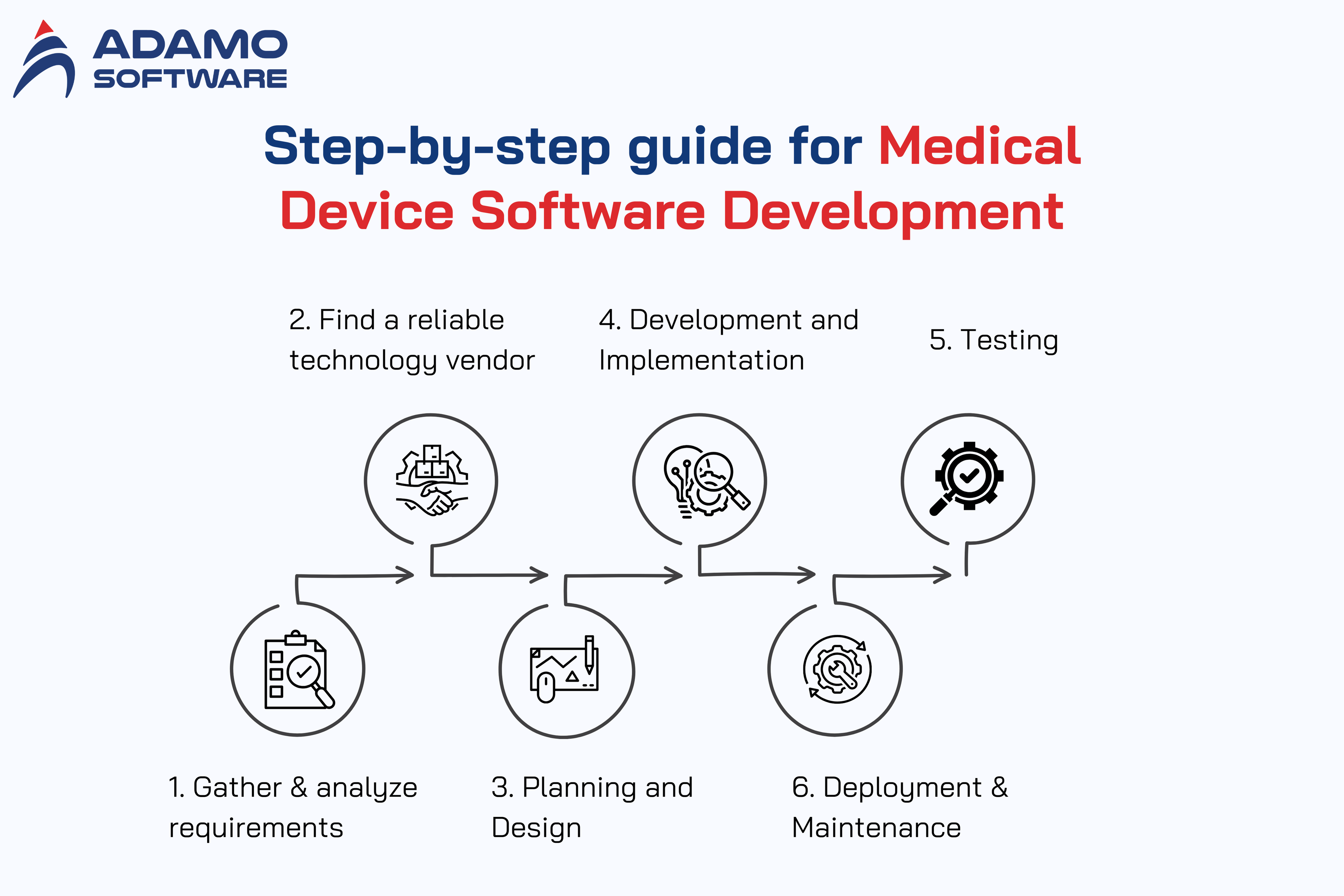
1. Gather and Analyze Requirements
Developing medical device software requires you to have a clear vision and roadmap. Therefore, before starting the development process, you must research the medical device software market and user requirements.
In addition, other important factors to consider before development include risk analysis, medical usability testing, and medical regulatory aspects.
2. Find a Reliable Technology Vendor
The next step is to find and select a trusted provider. Developing compliant medical device software on your own is almost impossible due to the increasing national and global data protection and security regulations. As well as comply with different regulatory standards on quality assurance, usability, and risk management.
It is best to partner with the right development partner to help you overcome those hurdles and beat your competitors in the market. Today, software development companies can guide you through the entire process from start to finish, from ideation to implementation and launch to the market.
3. Planning and Design
Project planning is an important step at this stage. You have to thoroughly analyze every aspect of the healthcare software such as user requirements, functional and non-functional needs, third-party integrations, and the development roadmap.
Additionally, UI/UX design is also an important step at this stage. Your UI/UX design team must develop an interface that focuses on user-friendliness and easy navigation for healthcare professionals.
So, it can be said that a careful planning phase allows your development team to set a realistic development timeline. It also estimates the project cost using a software development cost calculator and considers the important technical parameters for developing medical device software.
4. Development and Implementation
Once you have completed the preparation work, you will start developing the medical device software. Remember to prioritize quality, accuracy, and compliance with medical regulations at this important stage. In addition, your software must integrate seamlessly with the healthcare context to allow for smooth data exchange between different systems.
5. Testing
After successfully developing and deploying the medical device software, you need to rigorously test it to ensure the accuracy and efficiency of the software before it reaches the users. Testing can help you detect and fix errors in time.
Additionally, it enables you to verify the compliance of your medical device software with federal regulations and standards while still adhering to the user requirement specifications listed during the development phase.
Make sure you look for testers who understand the end user. In addition to simply following established test scripts, good testers will put themselves in the end user’s shoes. From there, they will suggest improvements that better suit the needs of each user and provide useful feedback so that users can get the most out of the product.
6. Deployment and Maintenance
The final step in the development process of medical device software is deployment and maintenance. After ensuring that all coding errors have been fixed during testing, the final code is deployed into the software and then deployed or delivered to the customer. Furthermore, after deployment, don’t forget ongoing support and maintenance to ensure the software works as expected and offers the highest performance on the market.
VI. Do you need help with custom medical device software development?

Developing medical device software is not easy because it requires compliance with strict regulations and changing trends. But with the right partner, these obstacles can become opportunities. At Adamo Software, we develop secure, scalable, and compliant medical software. Our healthcare software development team helps you create solutions that improve patient care and streamline workflows.
If you need a professional development team to help you develop custom medical device software or enhance an existing project, Adamo is here to help! Let’s build innovative medical software that sets you apart.





